Signifier® Medical Ushers In New Era of Treatment for Sleep Apnea and Snoring with FDA Authorization of eXciteOSA® Device
Revolutionary first-ever daytime treatment for mild obstructive sleep apnea and snoring will now be available in the United States
Boston, MA, USA, and London, United Kingdom
February 09, 2021
The FDA has authorized eXciteOSA, the revolutionary first-ever daytime treatment for mild obstructive sleep apnea and snoring. Used for only 20 minutes per day for a period of six weeks and then twice per week, the therapy is clinically proven to improve the quality of sleep by significantly reducing obstructive sleep apnea and snoring.
Signifier Medical Technologies, an innovator in the sleep disordered breathing market, today announced that eXciteOSA®, the first and only daytime intraoral neuromuscular stimulation device for treating mild obstructive sleep apnea and snoring, has been cleared by the U.S. Food and Drug Administration (FDA), giving millions of sufferers in the U.S. access to this innovative therapy.
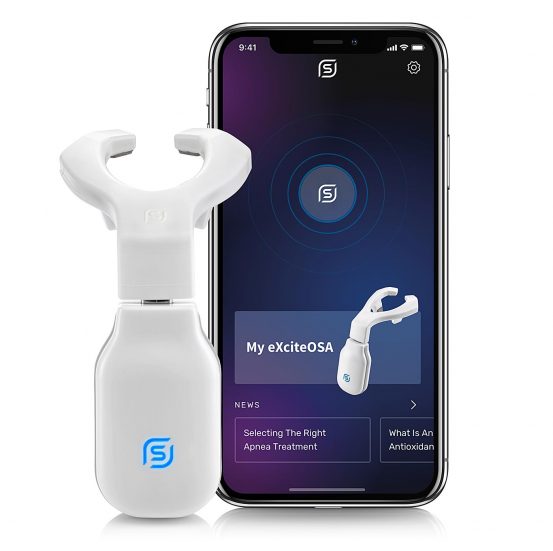
Unlike devices used while patients sleep, eXciteOSA is the first device used while awake that is intended to improve tongue muscle function, targeting the root causes of these conditions. Used for only 20 minutes per day for a period of six weeks and then twice per week, the therapy is clinically proven to improve the quality of sleep by significantly reducing obstructive sleep apnea and snoring.1-4
In a study of 65 mild obstructive sleep apnea patients, 79% of patients responded to therapy with a mean reduction of 52% in AHI (Apnea Hypopnea Index), a 50% reduction in ODI (Oxygen Desaturation Index), and a 3.9 point reduction in ESS (Epworth Sleepiness Scale). In the pivotal study of the device, eXciteOSA exceeded the targeted endpoint of a 20% mean reduction in snoring time, achieving nearly a 40% mean reduction in snoring time across 115 patients.
“We are thrilled that this groundbreaking treatment will soon be available in the U.S., offering an entirely unique, simple, and comfortable solution that has the potential to improve the lives of millions of people,” said Akhil Tripathi, CEO, and co-founder of Signifier Medical Technologies. “Restful sleep is critically important, yet for too many it is out of grasp because they or their partner suffer from a sleep disorder. We look forward to launching this revolutionary new option to select markets in the coming weeks.”
Obstructive sleep apnea and snoring affect one in three people – as many as 110 million Americans. Left untreated, obstructive sleep apnea can lead to serious complications such as hypertension, heart attack, glaucoma, diabetes, cancer, and cognitive and behavioral disorders.
“Treatment of sleep apnea has been shown to be highly effective for patients as there is growing evidence that it improves symptoms, reduces blood pressure, improves glucose control, and reduces the risk of motor vehicle accidents, among other benefits. A strong argument can be made that early treatment may be beneficial since the consequences of the condition may become irreversible,” said Atul Malhotra, M.D. Research Chief, Pulmonary, Critical Care and Sleep Medicine at UC San Diego Health, who served as a clinical trial investigator for the eXciteOSA device. “I believe that eXciteOSA will be an important treatment approach for patients with snoring and mild sleep apnea and look forward to offering it to my patients.”
Signifier Medical has developed a digital OSA therapy interface for patients and clinicians, which includes an intuitive app for use alongside the eXciteOSA device. The app is designed to give personalized care and motivate patients to achieve high therapy adherence. A “light touch design” with no fitting required, the eXciteOSA system includes digital connectivity between patient, bed partner, and provider to enable virtual setup, remote monitoring, and an overall exceptional patient experience.
“The FDA authorization for eXciteOSA is great news for OSA patients. This novel device will bring benefit to a neglected cohort of the sleep disordered breathing condition, namely those with mild obstructive sleep apnea,” said Peter Cistulli, M.D., Professor of Respiratory Medicine and Head of the Discipline of Sleep Medicine at the University of Sydney, and Head of the Department of Respiratory and Sleep Medicine at Royal North Shore Hospital.
About Obstructive Sleep Apnea and Snoring
Nearly 1 billion adults aged 30 to 69 years are estimated to have obstructive sleep apnea (OSA) globally. There is a strong, clinically proven link between OSA and co-morbidities like diabetes, hypertension, and strokes. Mild OSA affects over 110 million people in the US and 100 million people in Europe.
Obstructive sleep apnea is marked by the recurring collapse of the upper airways during sleep. The most common symptoms are restless sleep, snoring, tiredness during the day, decreased intellectual alertness, and personality alterations. Higher risks of cardiovascular diseases and increased mortality rates have been associated with OSA.
Standard therapy of all advanced levels of sleep apnea is with a continuous positive airway pressure (CPAP) device. Many patients find this therapy invasive, and the long-term compliance of CPAP therapy is limited, so there is a clear demand for new forms of treatment.
About Signifier Medical Technologies
Signifier Medical Technologies is a medical technology company focused on the development and commercialization of innovative and non-invasive solutions for patients with sleep disordered breathing conditions and snoring.
Signifier’s proprietary therapy eXciteOSA® is the first and only daytime genioglossal (tongue) muscle-neurostimulation technology authorized in the US for mild obstructive sleep apnea and snoring and is supported by clinical data from prestigious and well-recognized universities and academic institutions, that provides a safe and effective treatment for its patients.1-3
References
- White DP. Sleep-related breathing disorder: 2—pathophysiology of obstructive sleep apnoea. Thorax. 1995; 50:797–804. [PubMed: 7570420]
- Wessolleck E, Bernd E, Dockter S, et al. Intraoral electrical muscle stimulation in the treatment of snoring. Somnologie (Berl). 2018; 22 (Suppl 2): 47–52.
- Sama A, et al. Daytime Intraoral Neurostimulation with eXciteOSA® for Treatment of Snoring and Mild Sleep Apnea. CHEST Annual Meeting Notes. 2018.
- Clinical study of 115 patients with snoring or mild OSA (Apnea-Hypopnea Index (AHI <15 n=65)). Objective snoring and respiratory parameters were recorded with 2 consecutive WatchPAT® night sleep studies before and after the use of the device. An intra-oral tongue stimulator (eXciteOSA®) device was used for 20 mins, once a day for a 6-week period.
For media enquiries:
[email protected]
+44 (0) 207 096 0586

